Hiroyuki Mano, Hiromi Sasaki
Scirrhous gastric cancer progresses quickly and is often too late when it is discovered.Therefore, it was difficult to obtain a sample for research, and the development of a treatment method was delayed.This time, by using cells contained in the patient's ascites, a comprehensive genomic analysis of scirrhous gastric cancer was performed for the first time, and we succeeded in finding a therapeutic target.We spoke with Hiroyuki Mano, director of the National Cancer Center Research Institute, and Hiromi Sasaki, who conducted the research.
――What kind of cancer is scirrhous gastric cancer?
Mr. Mano: Scirrhous gastric cancer is an intractable cancer that accounts for 5 to 10% of all gastric cancers.It is a very severe cancer that spreads easily under the mucous membrane, and when it is diagnosed, it has already penetrated the stomach wall and metastasized to the peritoneum (peritoneal dissemination), and surgery cannot be performed in many cases.
Until now, for scirrhous gastric cancer, genomic analysis and research on carcinogenic mechanisms have not progressed much.In Europe and the United States, the incidence of gastric cancer is not as high as in Japan.Therefore, scirrhous gastric cancer has not received much attention in cancer genome projects centered on Europe and the United States, and rigorous analysis has not been performed.
Use cells obtained from ascites
――This time, you used cancer cells in ascites for your research.
Mr. Mano: Yes.The peritoneum is the membrane that surrounds the internal organs such as the stomach and intestines in the body.Cancer cells metastasize to the peritoneum and fluid collects in the peritoneal cavity (the space surrounded by the peritoneum).This is called ascites.
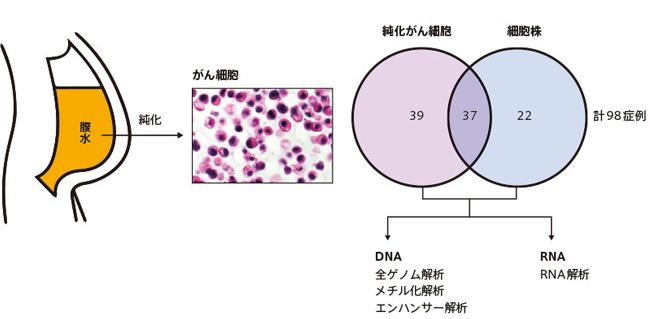
We were able to use the ascites of patients with scirrhous gastric cancer for research.Ascites contains cancer cells that have invaded from the stomach wall, mixed with various cells including blood cells.We analyzed the genome of the cancer cells.1.
――What made you decide to use cancer cells in ascites?
Mr. Mano: Dr. Sasaki has been collecting cancer cells from ascites for many years.In 2017, when I became the director of this institute, I met Dr. Sasaki and learned that cancer cells in ascites were being collected.I applied for joint research because I thought this would be a valuable opportunity to analyze scirrhous gastric cancer.
Mr. Sasaki: I still remember Dr. Mano's eyes shining when I explained my research (laughs).
――When did you start collecting?
Mr. Sasaki: It's from around 2010.I started collecting unique samples with the intention of collecting them for research.First, with the consent of each patient, only cancer cells are removed from the cells of ascites.These are purified cells.We also tried to establish cell lines.Whole cell pellets in ascites are cultured in normal medium and established.Established cells (cell lines) are convenient because they can be subcultured indefinitely.However, since the success rate of straining is about 30%, we performed this analysis including purified cells that had been cryopreserved in addition to the cell line.It can be confirmed by comparing the data of both that the characteristics of the established cells reflect the characteristics of the purified cells.
In this way, we were able to utilize the cancer cells of a total of 98 patients.
New discoveries by genome analysis
――What did you find out from the genome analysis?
Mr. Mano: We sequenced the entire genome of cancer cells from 98 patients using a next-generation sequencer.Then, we investigated what kind of acquired mutations (somatic mutations) were contained in the obtained cancer genome.
As a result, surprisingly, the scirrhous gastric cancer genome showed a high degree of "instability".In other words, it is a genome with an unusually high degree of gene amplification.This was a disappointing discovery.Scirrhous gastric cancer is classified as a part of diffuse gastric cancer, and the characteristic of diffuse gastric cancer is the "stability" of the genome.
Until now, the Cancer Genome Project has targeted surgical specimens for analysis, but since there are few indications for surgery for scirrhous gastric cancer, I imagine that the specimens were not available and tended to be excluded from the target. It is.In addition, fibrosis of the stomach wall is progressing in scirrhous gastric cancer, and even if a sample is obtained, it is expected that the proportion of cancer cells in it was low.As a result, it turned out that the cancer genome project so far did not accurately grasp the characteristics of scirrhous gastric cancer.
――Specifically, what kind of gene mutation did you have?
Mr. Mano: A number of particularly sophisticated gene amplifications were found in the genes of the "receptor tyrosine kinase-RAS / MAPK pathway" involved in cell proliferation control.Also I discovered before2There was also the EML4-ALK gene fusion, which is the cause of lung cancer.Gene fusion is a mutation in which two genes are linked.
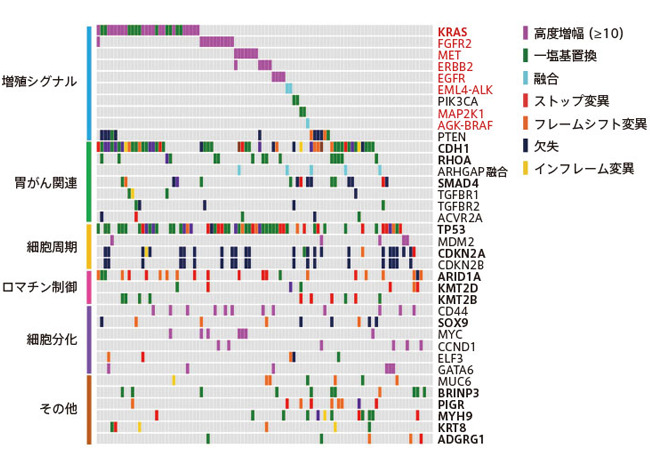
Many mutations such as gene amplification and gene fusion are also a characteristic of genome instability, and it is a nod to the characteristic of scirrhous gastric cancer that it is easy to metastasize.
I was also very pleased that another cancer molecular-targeted drug already exists for quite a few of the mutations found here.Molecular-targeted drugs target mutant molecules, so the effect of the drug is effective even if the type of cancer is different.Gene amplification and fusion were found in 50% of all cases of scirrhous gastric cancer, and half of them already had molecular-targeted drugs.
We actually conducted experiments with several molecular-targeted drugs and individual mice to confirm the efficacy of the drugs.We asked Sasaki Laboratory for experiments using cells and individuals.
--You also did transcriptome analysis.
Mr. Mano: Transcriptome analysis, enhancer analysis, etc. were performed in multiple layers, and the following was clarified.
First, scirrhous gastric cancer cells are divided into two groups according to the expression level of genes related to epithelial-mesenchymal transition (EMT). EMT refers to the acquisition of mesenchymal cell properties (infiltration, stray ability, etc.) by losing the original properties of epithelial cells (cell-cell adhesion ability, etc.) when cancer metastasizes. increase.
In the group with high EMT gene expression, a characteristic super-enhancer region (a place where enhancers that control gene expression are concentrated) was found.In the future, researching this super enhancer may be useful for the development of therapeutic agents and the elucidation of the mechanism of carcinogenesis.
Furthermore, it was found that transcription factors such as TEAD are activated as a result of increased expression of the EMT gene cluster. TEAD is known to be involved in organ sizing and carcinogenesis, and we have confirmed that TEAD inhibitors are effective in these gastric cancers.
Widespread application to cancer genomic medicine
――Since some of the existing molecular-targeted drugs and inhibitors are effective, they are expected to be applied to cancer genomic medicine.
Mr. Mano: As mentioned earlier, our analysis results show that one-quarter of scirrhous gastric cancers have gene mutations for which existing molecular-targeted drugs are effective.In the future, we would like to proceed to clinical trials within a year so that these existing drugs can be used for scirrhous gastric cancer.In addition, we have not received approval in clinical trials for TEAD inhibitors, so we would like to proceed with research and development.
Cancer genomic medicine began in 2019 in Japan.It is a medical treatment that examines what kind of mutation is in the cancer genome of a patient by a test method called "gene panel test" and uses the most suitable molecular target drug for that person.Since there are still few drugs that can be used, we hope that many researchers will actively carry out analyzes like this one, and that the number of patients who can be treated will increase as a result of the accumulated results.
--The genomic information of this research is open to the public.From that point as well, the development of research is expected.
Mr. Sasaki: Yes.The information is open to the public and can be used by anyone in consultation with the data registrant (lead author, Yosuke Tanaka, Researcher, National Cancer Center Research Institute).We believe that this research is extremely valuable as a research resource.This is because the cell line, genomic information, and clinical information are linked as a set.For cell lines, it can also be used for research and development, and such resources are called bioassets in the field of drug discovery.I think research on scirrhous gastric cancer will continue to develop in the future.
--What is the secret that made it possible to collect cancer cells and cell lines in ascites?
Mr. Sasaki: Is it interest and enthusiasm?Since I have been working for the National Cancer Center for a long time, it may have been possible to steadily carry out research on infrastructure development called cancer cell collection.Many young doctors who have trained in my laboratory are now working at core hospitals nationwide.Through their introduction, the patient's ascites was obtained and used in research.
It is important not to just blindly collect cancer cells, but to think about what is important in research.In addition, pancreatic cancer, which is an intractable cancer, and ovarian cancer, which is rapidly increasing in Japan, are also prone to ascites, so I also collected ascites from these cancers to obtain purified cancer cells and cancer cell lines. I am in stock.These genome analyzes are yet to come.
――What are your thoughts on this research?
Mr. Sasaki: I am really fortunate to be able to collaborate with Professor Mano and others.Even before that, I had partially done genome analysis.However, it was not possible to perform a comprehensive multi-layer omics analysis like this time, and therefore the research results were limited.
Genome analysis work can be outsourced, but in order to complete the subsequent data analysis, including several reanalysis, until the results are sufficient, it is necessary to carry out such joint research. I think it was impossible.
Mr. Mano: It would certainly be difficult to put together such research with only outsourced genomic data.We too were full of things that didn't work at first.As I tried various things, it led to new discoveries, and the next new discoveries were derived.
I had no choice but to try the effectiveness of the inhibitor one by one in an experiment, but in the end, I was glad that the results were directly linked to clinical practice.I have been involved in basic cancer research for a long time, and at the core of it has always been a strong desire to cure patients.So I'm really happy with the results this time.
-- thank you.
The listener is Yoshiko Fujikawa (science writer).
Author Profile

Hiroyuki Mano
Director, National Cancer Center Research Institute, Director, Department of Cellular Informatics
Graduated from the University of Tokyo School of Medicine in 1984.After working as an intern in internal medicine, he obtained a doctorate (medicine) in 1992.After working as a professor at Jichi Medical University School of Medicine and a professor at the Graduate School of Medicine at the University of Tokyo, he has been in his current position since 2016.

Hiroki Sasaki
National Cancer Center Research Institute Basic Clinical Development Research Core Center (FIOC) Researcher, Drug Discovery Targets and Seeds Search Division
Completed the Graduate School of Agriculture, University of Tokyo in 1990 (Doctor of Agriculture). After joining the National Cancer Center Research Institute (at that time) as a researcher in 1991, he became the director of the department and unit, and then the director of the FIOC department in 2013. He retired in 2020 and is in his current position.
References
- Tanaka, Y. et al. Nature Cancer 2, 962–977 (2021).
- 2 Soda, M. et al. Nature 448, 561–566 (2007).
* This article is reprinted from "Nature Digest".
Reprinted from: Nature Digest 2022 No. 1
"Therapeutic target seen in whole genome analysis of scirrhous gastric cancer'
Nature Digest Vol. 19 No. 1 | doi: 10.1038 / ndigest.2022.220130
