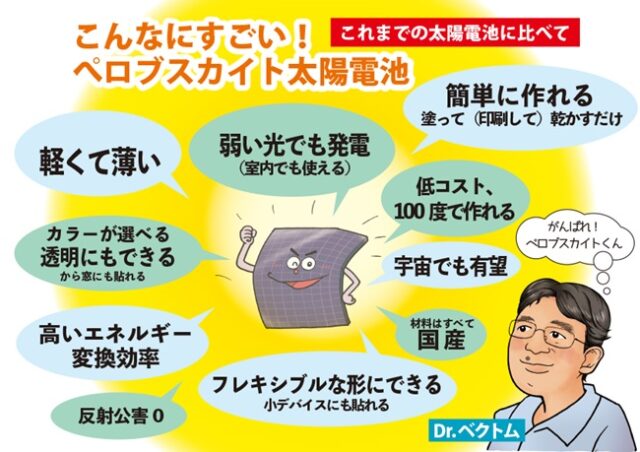
A turning point has arrived for solar cells, which play a central role in new energy. [Graph below: Solar installed capacity in each country per area (from Ministry of Economy, Trade and Industry data)]. Thin solar cells that replace silicon solar cells are attracting attention, especially those using crystalline films called perovskites, which are on the verge of commercialization. By using a material that is completely different from silicon, it has the potential to change the very nature of society. As the world situation becomes uncertain, expectations are rising that production can be made using only domestically produced materials*1.The government, which aims to increase the share of solar power generation to 2030 to 14% in 16, is calling for ``early commercialization.'' *2 While waving the flag, major electronics, chemical, and housing manufacturers are also preparing their production systems with an eye on expansion from 2025.
Professor Chikara Miyasaka, who is in the spotlight as the inventor of perovskite solar cells, looks back on his career as a university researcher → corporate researcher → university teacher and venture manager, his research and development up to now, and the academia ecosystem. We also asked him about his message to high school students.
*1 Japan's energy self-sufficiency rate is said to be around 10%, near the lowest among OECD countries.
*2 The "Basic Energy Plan" approved by the Cabinet in October 2021 sets a target of 10-2030% renewable energy in the power source mix for 36, of which 38-14% will be solar.
Also see the 2023th meeting of the Ministry of Economy, Trade and Industry's Green Power Promotion Working Group, August 8, 31.
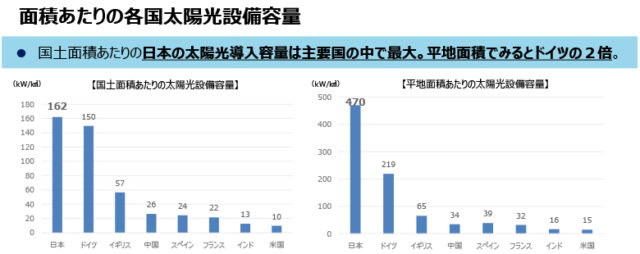
(From Ministry of Economy, Trade and Industry materials)
What exactly is perovskite?
Is it the name of a mineral?
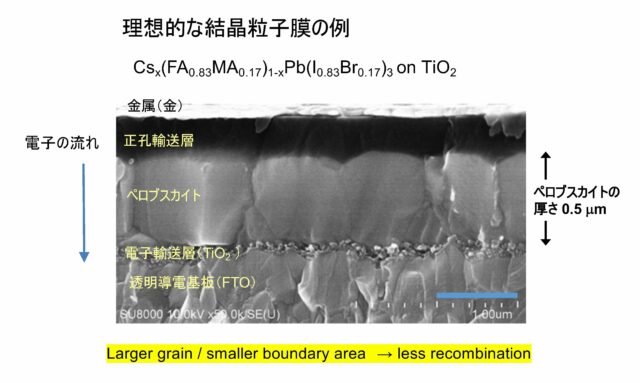
Originally, it was named after its discoverer, Lev Perovsky (Russian nobleman and mineralogist: 1792-1856). The main component is calcium titanate (CaTiO3). It is an inorganic compound made of calcium, titanium, and oxygen. Because it has an unusual structure [lower image on the left (the image on the right shows an actual perovskite solar cell]), it is a general term for substances with the same crystal structure, and perovskites made of oxides of these metals exhibit strong dielectric properties. It is unique in that it is commonly used in print heads of inkjet printers. On the other hand, instead of oxides, there are perovskites made of halogens such as iodine (I) (for example, CsPbI3). These can be synthesized artificially, and some generate electricity when they absorb light.

Since this halogenated perovskite dissolves in solvents, it can be used for power generation by applying the dissolved raw material and drying it to form a thin film. For example, thin and light perovskite solar cells can be created by applying raw materials to plastic films, etc., and by installing these in various places in daily life, they can generate electricity with high conversion efficiency when exposed to light. . [Bottom image]
The composition and manufacturing method are completely different from the currently popular solar cells using semiconductor silicon, and their characteristics are compared as shown in the illustration at the top.
It seems promising just by looking at it, but what kind of things should we learn in order to research it?
The research and development field is called photoelectrochemistry. It is an area born from ``electrochemistry'' in the field of ``physical chemistry'' in chemistry, and means electrochemistry that involves light. A typical example is the photolysis of water using semiconductors as electrodes. By the way, the English notation is Photoelectrochemistry. Pexel Technologies Co., Ltd., a venture company launched in 2004, has the initials ``Cell'' added to it.
Invention of perovskite solar cells
Simply pasting or printing materials onto a glass plate or plastic film seems like a far-fetched idea, but when, where, and who came up with it?
Let's talk about the steps leading up to invention. I spent my graduate school doctoral studies in the laboratory of Professor Kenichi Honda*1, who is famous for his work on photocatalysts, and after completing my studies, I got a job at one of Japan's leading film manufacturers. He was involved in research on simulating plant photosynthesis using photoelectrochemistry. However, since it was a company research institute, I was made to do everything. Research and development of artificial retinas and lithium-ion secondary batteries are representative examples. Although they were not commercialized, they both elucidated the principle and were published in Science. Of course, it's not my strength alone. During this time, I have some painful memories, such as my fully prepared research being interrupted due to company circumstances. Well, since you're a member of an organization, it's only natural that you follow company orders.
This is different from university research. So, change jobs?
Eventually, after turning 45, he started considering a career change, and the topic he was given at that time was dye-sensitized solar cells. His idea was to create solar cells using dye sensitization technology, which is used to increase the sensitivity of silver halide photography.His idea was to create solar cells using dye sensitization technology, which is used to increase the sensitivity of silver halide photography.He is a representative of solar cells made using chemistry, and is a predecessor of perovskites in terms of power generation mechanisms. He just wasn't very keen on it personally. Because he uses liquid (electrolyte), he expected that there would be problems with durability due to leakage. The inventor is Professor Michael Gretzel*2. He published a paper in 1991 and is still continuing his research, and has recently increased the conversion efficiency to 14%. The value is enough to run devices that don't require much electricity, and it has even been commercialized.
*1 From 1925 to 2011, professor at the University of Tokyo, professor at Kyoto University, professor at Tokyo Polytechnic University, president of the same, known for the ``Honda-Fujishima effect'' in 1972. He focused on the photocatalytic properties of titanium oxide and led a number of discoveries and inventions.
*2 Michael Grätzel: 1944-Professor, Swiss Federal Institute of Technology Lausanne
How does this lead to perovskites?
As some of you may have noticed from the story so far, photocatalysis, photosynthesis, and dye sensitization all convert light energy into chemical and electrical energy through redox reactions. Photosynthesis converts carbon dioxide and water into glucose, and photocatalysts use titanium oxide as a semiconductor to split water into oxygen and hydrogen. Dye-sensitized solar cells and perovskite solar cells convert light directly into electrical energy. Titanium oxide is also used here to transport electrons. The former uses a pigment to absorb visible light (titanium oxide only absorbs ultraviolet light), and the latter uses perovskite as a semiconductor that absorbs visible light and generates electricity, and by coating and printing this on the electrodes, the battery is activated. can.
It's all connected!
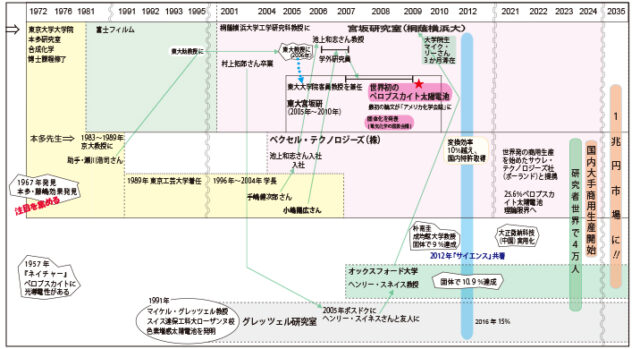
Immediately after I quit my job and moved to this university, I started the venture company I mentioned earlier, and I was also researching dye-sensitized solar cells both in the laboratory and there. Then a young man appeared in front of me who wanted to use perovskites instead of dyes. Haruhiro Kojima was a master's student at Tokyo Polytechnic University, which has a long tradition of photographic technology education in Japan, and was researching perovskites. The person who introduced me was Kenjiro Teshima, who was a teacher at Tokyo Polytechnic University, applied for a job at Pexel, and joined the company. When I heard Kojima's story, I accepted him and said, ``I don't know what it is, but it has an optical function, and it's a method that no one has tried before, so why not try it out?'' I decided to have him come.
I was quite disappointed. This is something that would be unthinkable at a traditional or large-scale university.
Yes, this place is small, so it's flexible. Also, there are many children who have failed their entrance exams, so I thought it would be an inspiration to them. Of course, she also felt a strange connection. The president of Tokyo Polytechnic University at the time was Kenichi Honda. After retiring from Tokyo University, he transferred to Kyoto University, and then returned to Tokyo.
Under my guidance, Mr. Kojima quietly continued experiments using perovskite, a material that everyone thought had little potential. What's more, after graduating from my master's degree, he entered the doctoral program in the laboratory that I now have at the University of Tokyo.
In 2009, the third year of my doctoral course, I used perovskites to increase energy conversion efficiency to 3.8%, and co-authored a paper with myself on the world's first solar cell using perovskites. ☆mark]. Kojima published a dissertation on this perovskite research and received his Ph.D.
More breakthroughs
Almost 15 years have passed since then, and it is said that there are currently 4 researchers working on perovskite solar cells, but has it come so easily?
not yet. Perovskite solar cells have now caught up to the light conversion efficiency of silicon, but another breakthrough was needed to raise that from just under 4% at the time.
what kind? And who exactly?
Initially, we used a liquid containing iodine to transport charges, similar to dye sensitization. However, this had the problem that some of the perovskite would dissolve into it and the efficiency would not increase.
Does that mean that the perovskite will break down in the electrolyte?
Well, you can say that. No matter how efficient this is, it is not practical. Kojima was also aware of this, and in 2008 he suggested the possibility of solid matter. However.
Is there some new development happening?
Please see the chronology above for details. My research background will be the main focus, but the story will expand from Toin Yokohama University to Switzerland, England, South Korea, China, and Poland. The next protagonist is again a young man, but this time he's British. Henry Snaith*3 was researching whether solid charge transport materials could be used in dye-sensitized solar cells. When he was working with Professor Michael Gretzel, he happened to become friends with Takuro Murakami*4, who happened to be a postdoc from our laboratory, and learned about perovskites. After that, he got a job at Oxford University, and perhaps he was so interested that he sent a graduate student to my lab for three months to learn how to make perovskites. And that was exactly one year later. His laboratory achieved a conversion efficiency of 3% (see figure below).
What, what did you do?
I tried converting a liquid electrolyte into a solid form, which is my specialty. The world was astonished by this high efficiency, and everyone said, ``This can be used!'' The paper we co-authored attracted attention, and those involved at the time went on to jointly receive several prestigious awards worldwide*5. And in the blink of an eye, researchers who had previously worked on dye-sensitized solar cells became perovskite researchers!
Since then, research has progressed rapidly.
Our laboratory has also obtained a patent, and the world has worked hard to increase conversion efficiency, and now we have achieved over 26%, almost the same as silicon. The only thing left to do is to create a concrete product, so-called implementation.
*3 Henry Snaith: Current professor at Oxford University
*4 Current leader of the Organic Solar Cell Research Team, National Institute of Advanced Industrial Science and Technology (AIST)
*5 Received numerous awards, starting with the Clarivate Analytics Citation Honor Award in 2017, as well as the Rank Award in 2022 and the Asahi Award in 2024.
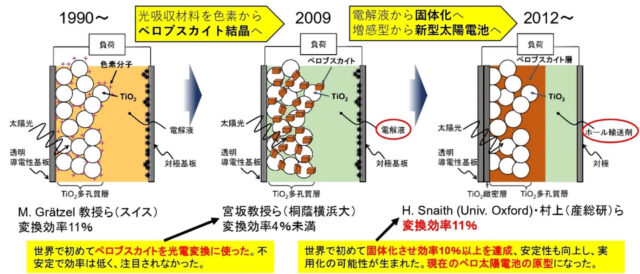
From dye sensitization to perovskites (from AIST materials)
To Japanese companies and the young people who aim to work there
However, there seems to be a problem. I wonder if Japanese companies are lagging behind. Perovskites are full of good things, even though they are ahead of the curve in material development.
After all, large companies only do what makes them profitable. There is no need to take risks while you are making money. I experienced this many times in my previous job. Moreover, among Japanese people, there are many people who cross stone bridges by hitting them, and others who do not cross the bridge even if they hit it. This is one of the reasons why Japan is often overtaken by Europe, America and China.
Another issue is the trauma of silicon solar cells. Initially, Japan boasted an overwhelming market share, but was overtaken by South Korea and China. Regarding perovskite, there is a widespread preconception among the management that ``since it is the same solar power generation, we will lose again.'' I would like people who are looking to work at large companies to reverse this culture. I won't fail this time.
How to choose a laboratory
I have seen that people grow around him, and students and young researchers from different backgrounds, such as Mr. Kojima, Mr. Kazushi Ikegami, Mr. Takuro Murakami, and Mr. Kenjiro Teshima, took center stage and relationships at important times. He was active under. It might be a good idea to include Koji Segawa, who was invited to the University of Tokyo as a professor.
I agree. He was an assistant to Professor Honda at Kyoto University, and when I became a professor at the University of Tokyo, he recommended me to be a visiting professor at the University of Tokyo.
I love seeing happy faces of students wherever I go, and I've done everything I can to make that happen. Even undergraduate students are taken to overseas academia. Then, when I go there, I become a catalyst and meet many different people. Some people will grow up because of this.
His motto is ``Personnel, inspection, and effort'' is said to be his motto.
It is a play on the old expression, ``Do your best in one's affairs and wait for the destiny.'' ``Human resources'' is about gathering people and meeting people, which is very important whether it's a company research lab or a university. People invite others, the circle expands, and results are produced. By the way, ``inspection'' means to thoroughly investigate and find something good.
I once heard the phrase, ``Research is a human relationship that revolves around the truth.''
Message to high school students
During high school, it is of course important to learn broadly and shallowly, but I also want to have at least one thing that I learn deeply. There are also classes such as ``Comprehensive Inquiry Time'' and ``Science and Mathematics Inquiry'', so it's easy to learn about methodologies and methods. When you think, "What's going on with this?", start investigating from there. Also, even if it seems unrelated to the theme at first glance, if you can reach it with your hands, try it first. Then research thoroughly until you are satisfied. During an experiment, if you come across something that makes you wonder, ``Huh?'' I want you to stop and think about the cause. If you ignore it in your haste, you may miss a big discovery.
It's like ``put in the effort and wait for the results.'' Through this process, you will also find out what you are interested in, and even if you are unfortunately unsuccessful, knowing that is also a great accomplishment.
A little off-topic, but I still play the violin in my spare time between research and research it as a musical instrument. I decided to submit my new discovery to an authoritative journal, and it has been published three times so far.
During his junior high and high school years, he suffered a setback while studying for entrance exams, but he recovered as he entered university and graduate school. His graduate school years were particularly fulfilling, and he wrote many papers, including ones that were published in Nature and Science. I think that my previous ``pursuit of why'' and ``pursuit of love'' blossomed. This is something that still supports me even now.
Lastly, please give us a few words about generative AI.
AI draws conclusions based on a huge amount of information (big data), not thinking. My view is negative because relying on AI risks weakening people's ability to think with effort. It would be fine if AI was used only for advanced processing of information.
Thank you all!

Toin Yokohama University Specially Appointed Professor
Mr. Chikara Miyasaka
Graduated from the Department of Applied Chemistry, Faculty of Science and Engineering, Waseda University in 1976, and completed the doctoral program in Synthetic Chemistry, Graduate School of Engineering, University of Tokyo in 1981. During this time, he was a visiting researcher in the Department of Biophysics at the University of Quebec in Canada from 1980 to 81. Joined Fuji Photo Film Co., Ltd. in April 1981, Researcher at Ashigara Research Institute, December 4 - March 2001: Professor, Graduate School of Engineering, Toin University of Yokohama, April 12: Specially Appointed Professor, Faculty of Medical Engineering, Toin University of Yokohama, October 2017: Fellow, Research Center for Advanced Science and Technology, University of Tokyo; April 3-March 2017: Visiting Professor, Graduate School of Advanced Science and Engineering, Waseda University. He has won numerous awards, including the Asahi Prize in January 4 and the UK Rank Prize in July 2017. He graduated from Waseda University High School.
