Original: Nature (2021-11-08) | doi: 10.1038 / d41586-021-03025-0
How protein-based COVID vaccines could change the pandemic
Protein vaccines developed by biotechnology companies such as Novabax will soon be available.Scientists say there are many advantages.
carmengabriela / iStock Editorial / Getty
Pamela Sherry wants to be vaccinated against the new coronavirus infection (COVID-19).But she has been procrastinating.
"I believe the vaccine works. I want to prevent it," she says.However, because he is prone to allergic reactions and suffers from cardiovascular disease, he is worried about vaccination with the messenger RNA (mRNA) vaccine and viral vector vaccine used in the United States.Although these vaccines are safe for most people, they can, very rarely, cause serious adverse reactions such as myocarditis and thrombosis (October 2021).COVID vaccine and thrombosis: what we have learned so far, December 2021 issue "Six months after the start of COVID vaccination, what I learned about the vaccine"reference).
That's why she's waiting for more vaccine options.What she especially hopes for is a vaccine made by purifying viral proteins.Unlike mRNA and viral vector vaccines, which are manufactured using relatively new technologies, protein vaccines have been used for decades to protect people from viral infections such as hepatitis and shingles. It has a history.Protein vaccines can provoke a defensive immune response by directly administering the viral protein itself with an immunostimulant called an adjuvant, rather than administering the gene fragments needed to make the cells synthesize the viral protein. Can be done (June 2020 issue "Coronavirus vaccine development race"reference).
Although protein vaccines against COVID-19 have not yet become widespread, data from late-stage clinical trials have shown that they have few adverse reactions and have a strong protective effect, which is promising.Sherry, who sells stationery online at her home in Prosper, Texas, says, "I want to get such a vaccine as soon as I get it."
Sherry is likely to be vaccinated soon.A few months behind schedule due to quality control issues and manufacturing delays, but by the end of the year, a protein vaccine approval application was filed, according to an executive at biotechnology company Novabacs, Gaithersburg, Maryland, USA. It is finally ready to submit to the US drug regulator.Two Asian vaccine manufacturers, Clover Biopharmaceuticals (Chengdu, China; hereinafter Clover) and Biological E (Hyderabad, India), will also apply for approval to regulatory agencies in each country within the next few weeks or months. It is planned to do it.
Already, in some countries like Cuba and Taiwan, vaccination with domestic protein vaccines is underway.If protein vaccines become widely available in the future, the anxiety of people who are hesitant to inoculate like Sherry will be eased, and they will be able to be used for booster inoculation (additional inoculation), leaving a gap in the global pandemic response. May help fill.
The Coalition for Epidemic Preparedness Innovations (CEPI) has invested more than $ 5 billion in five protein vaccines currently under development. "Protein vaccines will bring a new era to COVID-10 vaccination," said Nick Jackson, head of vaccine development programs and innovative technology development at CEPI.
In essence it takes time
From the early stages of anti-pandemic measures, researchers expected that the development of protein vaccines would be delayed compared to the development of vaccines using other technologies.
We know how to use genetic engineering techniques to make mammalian, insect, and microbial cells produce large amounts of purified proteins.However, there are many steps in the process, and each step must be optimized for the target protein."It's essentially a time-consuming process," said Christian Mandl, a former vaccine maker executive and now consulting on vaccine development.Most of the protein vaccines currently under development are based on the spiked protein of the new coronavirus SARS-CoV-2 (see the figure "Basics of Protein Vaccines").
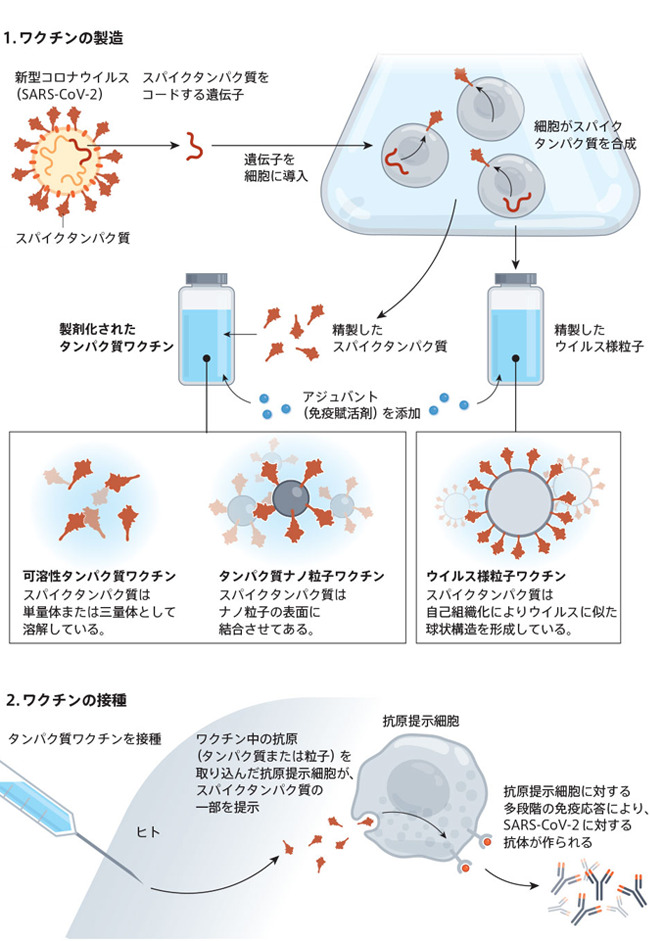
There are many methods for formulating a protein vaccine against COVID-19, such as a method using a soluble protein and a method using a protein bound to nanoparticles.Most protein vaccines are based on the coronavirus spike protein, but some vaccines use only important regions of the spike protein called the receptor binding domain.
Large-scale trials conducted by Nuvaxovid and Clover have already provided valid data. According to a preprinted paper published in October 2021, a 10-person trial conducted in early 2021 when the highly infectious delta variant was not yet prevalent, but a Novabax protein. The vaccine was said to be more than 3% effective in preventing the onset of COVID-90 (LM Dunkle et al. Preprint at medRxiv). https://doi.org/g5w92021).
On the other hand, Clover's protein vaccine is reported to have a COVID-19 onset prevention effect (not considering the severity) of only 67%.However, the reason for this low value is thought to be that the test was conducted after the epidemic of highly infectious mutant strains including the Delta strain.All vaccines yielded antibody levels comparable to the RNA vaccine, which is considered to be the most effective in this pandemic.
Ryan Spencer, CEO of Dynabacs Technology, Inc. (Emeryville, Calif., USA), the manufacturer of the adjuvant added to Clover's vaccine, said from clinical trial results that "for COVID-19" Protein vaccines are not a low-quality approach just because they take a long time to develop. "
Protein vaccines also seem to be safe.None of the more than 50 protein vaccines currently in clinical trials worldwide have caused serious adverse reactions. Vaccine reactions such as headache, fever, nausea, and chills that are common with mRNA and viral vector vaccines have also been found to be very infrequent with protein vaccines."Maybe it alleviates the anxiety of many," said Cindy Gay, an infectious disease physician at the University of North Carolina at Chapel Hill School of Medicine, who co-sponsored the Nuvaxovid vaccine trial.
However, even if one protein vaccine is confirmed to be effective and widely used, not all protein vaccines are.
One of the reasons is that the usage patterns of peaplomers differ greatly depending on the vaccine. Some vaccines use only one type of spike protein, while others use a combination of three.Also, some vaccines use the full length of the spike protein, while others use only fragments.
In addition, the cells used for production also vary from vaccine to vaccine.Vaccines from Novabax, Sanofi, a major pharmaceutical company, and GlaxoSmithKline (GSK) use moth cells called Spodoptera frugiperda to synthesize proteins, but Clover and Medigen. -Vaccine Biologics (High-end epidemic seedling biologics; Taipei, Taiwan) uses ovarian cells of Chinese hamsters, which are often used to produce therapeutic antibodies.In addition, the adjuvants added vary from vaccine to vaccine, and each stimulates the immune system in a unique way, resulting in different responses to the vaccine.
Thomas Breuer, GSK's Chief Global Health Officer, says all of these factors can lead to different efficacy and safety profiles for different vaccines. "It's easy to imagine the difference, but the specific difference will be clear from the results of Phase III trials and actual experience."
In wealthy countries, where the majority of the population has already been vaccinated, these results can influence booster vaccination plans.Currently, mRNA vaccines are often used as boosters, but if protein vaccines become available, more people may want them because of their tolerability.
Elimination of injustice
If the protein vaccine is approved, it is expected that the supply shortage, which is an obstacle to vaccination in low-income countries, can be quickly resolved.For example, Novabax and Clover have pledged to provide COVAX, a framework for the equitable distribution of vaccines around the world, with hundreds of millions of protein vaccines free of charge by the end of 2022.
Researchers specializing in global health argue that developing countries need to be able to produce their own vaccines in order to achieve fair distribution of the COVID-19 vaccine.To that end, more researchers should consider a simple, inexpensive production system that manufacturers in developing countries can quickly adopt, says Christopher Love, a chemical engineer at the Massachusetts Institute of Technology (Cambridge, USA). Points out.
Biological E uses a yeast-based system to produce vaccines licensed from Baylor College of Medicine (Houston, Texas, USA).According to Maria Elena Bottazzi, a virologist at Baylor College of Medicine who was involved in the development of this vaccine, it is "probably the easiest and cheapest" of the COVID-19 vaccines that have been or are about to be approved. It is said that it can be manufactured.
Ralf Clemens, a scientific adviser to Clover, who has long been involved in the vaccine industry, said vaccines like the mRNA vaccine had the advantage of speed in the early stages of the COVID-19 pandemic.But the upcoming protein vaccines have more advantages, he says. "I think that protein vaccines will eventually become the mainstream vaccines to protect the world from COVID-19."
(Translation: Yoichi Fujiyama)
* This article is reprinted from "Nature Digest".
Reprinted from: Nature Digest 2022 No. 1
"Will Protein Vaccines Converge the COVID-19 Pandemic?'
Nature Digest Vol. 19 No. 1 | doi: 10.1038 / ndigest.2022.220110
